Updates for Nirsevimab Effectiveness in RSV Prevention

I'll never forget when I first started working in a pediatric hospital (too many years ago now), the ICU and medical floors were filled with kids sick with respiratory syncytial virus (RSV). It was a little shocking to me; we didn't learn much about it in pharmacy school (it's a virus, and at that time, there wasn't much drug therapy for RSV), and I wasn't a parent yet, so I wasn't familiar with the yearly RSV scares.
RSV infection is widespread, but thankfully, new preventive therapies significantly reduce the risk of severe illness and hospitalization in infants.
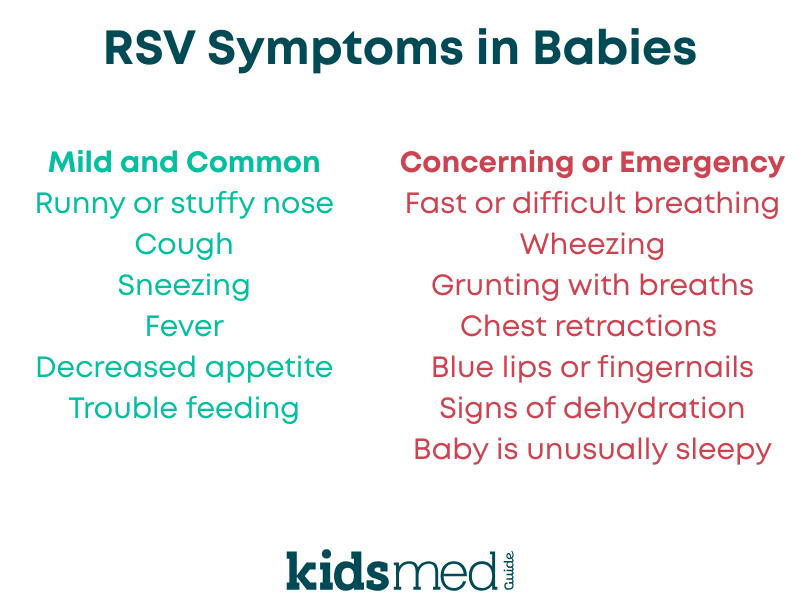
Many kids handle RSV infection with no serious long-term effects, but in some babies, it can be severe and sometimes life-threatening.
Nirsevimab (Beyfortus®) is an RSV antibody doing exactly what we hoped it would do: preventing many infants from ever getting that sick in the first place!
In this guide, we'll walk through what RSV is, how nirsevimab RSV prevention works, and what the latest 2024–2025 data are telling us about RSV prevention for infants in 2025. I'll aim to give you clear, up-to-date information you can discuss with your child’s pediatrician.
Understanding RSV and Its Effect on Infants
RSV is a respiratory virus that infects the nose, throat, and lungs. In older children and adults, it often causes a cold, but in infants, it can lead to more serious lower respiratory infections, such as bronchiolitis or pneumonia.
RSV itself makes babies miserable - fever, congestion, and feeling really crummy. Severe cases that lead to bronchiolitis or pneumonia may sometimes require professional medical support in the hospital setting with oxygen, IV fluids, or even intensive care. RSV has long been one of the leading causes of hospitalization in infants in the United States.
RSV activity tends to rise in the cooler months. The CDC’s respiratory virus data show RSV as one of the key viruses they track alongside flu and COVID-19, with seasonal surges that can strain pediatric hospitals. Activity usually peaks in the colder months of December and January.
Because RSV is so common and so contagious, and can have very real and dangerous consequences, RSV prevention for babies has become a major focus.
For RSV prevention for infants in 2025-2026, experts now recommend that all babies have protection either through a maternal RSV vaccine during pregnancy or through an RSV monoclonal antibody given directly to the baby. Most infants need one approach, not both.
What Is Nirsevimab and How Does It Work?
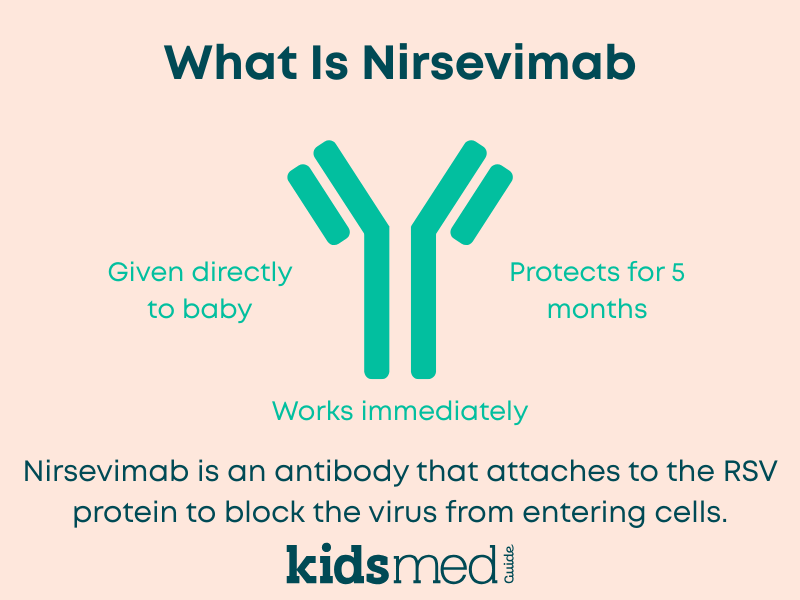
Nirsevimab is a lab-made antibody designed to recognize and neutralize RSV. It is not a traditional vaccine. With a vaccine, your child’s immune system learns to make its own antibodies over time. With nirsevimab, your baby is given ready-made antibodies that start working right away. This is called passive immunization.
A few key points about nirsevimab RSV prevention:
- It is given as a single injection in the thigh, usually before the RSV season starts or within the first week of life if your baby is born during the RSV season.
- Protection is expected to last at least 5 months, which aligns with a typical RSV season.
- In the United States, nirsevimab is recommended for most infants under 8 months of age entering their first RSV season, and for high-risk children aged 8 through 19 months entering a second season.
Other RSV antibodies exist, but this article will focus on nirsevimab for RSV prevention, as it has the most robust evidence and is widely used in the U.S.
Latest Updates on Nirsevimab Effectiveness
When nirsevimab was first approved, initial recommendations were based on clinical trials. The MELODY phase 3 trial in healthy late-preterm and term infants found that a single dose of nirsevimab reduced medically attended RSV lower respiratory tract infection by 74.5 percent compared with placebo.
The MEDLEY study then looked at infants with conditions like prematurity, chronic lung disease, or congenital heart disease—children who are at higher risk of severe RSV. In these infants, the safety profile of nirsevimab was similar to that of palivizumab, and serum levels were comparable to those associated with protection in the MELODY trial.
Since then, more real-world data have become available.
- A large U.S. study found that nirsevimab was 77 percent effective in preventing RSV-related emergency department encounters and 98 percent effective in preventing RSV-related hospitalizations during the 2023–2024 season.
- A 2025 systematic review and meta-analysis in The Lancet Child & Adolescent Health pooled real-world studies. They found that nirsevimab was associated with about an 83% reduction in RSV-related hospitalizations and a similar reduction in ICU admissions.
- Coverage in The American Journal of Managed Care in late 2025 highlighted that nirsevimab RSV prevention continued to significantly reduce infant RSV hospitalizations in the 2024–2025 season, with effectiveness similar to the first season it was used.
Taken together, these results support the continued use and recommendation of nirsevimab as a highly effective tool for RSV prevention in infants in 2025, not only in controlled trials but also in real-world pediatric practice.
Safety and Possible Side Effects of Nirsevimab
Any medication or immunization can cause side effects, and it's important to understand the risks and side effects and know what questions to ask your trusted pediatrician.
- In clinical trials and in real-world studies, the overall rate of adverse events was similar between babies who received nirsevimab and those who received a placebo.
- In high-risk infants (prematurity, chronic lung disease, or congenital heart disease), nirsevimab had a safety profile comparable to palivizumab, which has been used in pediatrics for many years.
The most common side effects reported were:
- Mild redness, swelling, or tenderness where the shot was given
- Temporary fussiness
- Occasional low-grade fever
These mild symptoms typically resolve on their own. Severe allergic reactions are rare but can occur. If your baby has trouble breathing, facial swelling, or seems severely unwell after any medication or immunization, call 911 or seek emergency care right away.
Before giving nirsevimab, your child’s pediatrician should review their medical history, including any prior reactions to medications or antibodies, prematurity, and underlying heart or lung conditions. That one-on-one discussion is the best place to weigh the benefits and any potential risks for your individual child.
How Parents Can Prepare for the RSV Season
Even with nirsevimab and other available RSV antibodies, RSV will not disappear entirely. Nirsevimab is very effective at reducing the risk of severe RSV illness and hospitalization, but it's not 100% protective.
- Plan ahead with your pediatrician. If your baby will be born during RSV season (typically fall and winter), ask in advance whether nirsevimab can be given in the hospital or soon after birth. If your baby is already home, ask whether they qualify based on their age, health conditions, and whether you received the maternal RSV vaccine during pregnancy.
- Reduce exposure when RSV is circulating. During peak RSV activity, try to avoid crowded indoor gatherings for very young infants, especially around people who are sick. The CDC’s respiratory virus dashboards can give a sense of when RSV activity is rising in your area.
- Stick with everyday hygiene habits. Frequent handwashing, covering coughs and sneezes, and cleaning high-touch surfaces may sound boring, but they do help prevent illness!
- Keep up with other vaccines. Protecting your baby and your household from flu and COVID-19 can reduce overall strain on your child’s immune system and on the healthcare system during respiratory virus season. (My 1-year-old once got Covid and RSV at the same time, and it was the sickest I have ever seen him!)
Related: Fever Reducers for Kids
Related: OTC Cold Medication Safety and Guidance
Related: Managing Cold and Flu Season at Home
Conclusion
Some folks might call advancements in RSV prevention the greatest thing since sliced bread. High-quality trials show that a single dose of nirsevimab significantly reduces severe RSV infections, and growing real-world data confirm large drops in RSV-related hospital and emergency visits. This means fewer sick babies, fewer hospital stays, and fewer life-threatening RSV cases!
For RSV prevention for infants in 2025-2026, major health organizations recommend that all babies be protected either with a maternal RSV vaccine during pregnancy or with nirsevimab after birth during their first RSV season, and during their second RSV season if high risk.
Deciding whether or not to give your child any medication can be scary. I encourage parents to ask their pediatrician for guidance and talk through any concerns. For what it's worth - having seen many children hospitalized with RSV, I would choose to protect my own kids with nirsevimab if they were babies (they're too big now!)
FAQ Section
What makes nirsevimab different from a vaccine?
Traditional vaccines teach the immune system to make its own antibodies over time. Nirsevimab is a ready-made antibody that is given directly to your baby. Protection starts right away, without waiting for the immune system to respond. This is especially helpful for newborns and young infants.
How long does one dose of nirsevimab protect my baby?
Current guidance and clinical data suggest that a single dose of nirsevimab protects for at least 5 months, which is about the length of a typical RSV season.
Is nirsevimab safe for premature infants?
Yes. The MEDLEY study specifically looked at preterm infants and those with chronic lung disease or congenital heart disease. In those groups, nirsevimab had a safety profile similar to palivizumab, the older monthly antibody that has been used for years in high-risk babies.
When is the best time to get nirsevimab for my baby?
The ideal timing depends on when your baby is born and when RSV season starts in your area. In the U.S., RSV season usually begins in the fall and often peaks in December or January, though timing can vary by location and year. For most families in the U.S., nirsevimab is given shortly before RSV season or within the first week of life if the baby is born during RSV season. Your pediatrician will use CDC and AAP guidance to pick the best timing for your baby.
Are there any side effects parents should watch for?
Most side effects are mild, such as soreness at the injection site, temporary fussiness, or a low-grade fever. Serious reactions are rare but include allergic reactions and require immediate medical attention. If your baby has difficulty breathing, swelling of the face or lips, or appears severely unwell after any shot, seek emergency care. Clinical studies to date have not shown major new safety concerns with nirsevimab in either healthy or high-risk infants.
The following references were used to compile this information:
CDC. (2025a, August 21). RSV Immunization Guidance for Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html
CDC. (2025b, September 26). Respiratory Virus Activity Levels. Respiratory Illnesses. https://www.cdc.gov/respiratory-viruses/data/activity-levels.html
Domachowske, J., Madhi, S. A., Simões, E. A. F., Atanasova, V., Cabañas, F., Furuno, K., Garcia-Garcia, M. L., Grantina, I., Nguyen, K. A., Brooks, D., Chang, Y., Leach, A., Takas, T., Yuan, Y., Griffin, M. P., Mankad, V. S., Villafana, T., & MEDLEY Study Group. (2022). Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. The New England Journal of Medicine, 386(9), 892–894. https://doi.org/10.1056/NEJMc2112186
Hammitt, L. L., Dagan, R., Yuan, Y., Cots, M. B., Bosheva, M., Madhi, S. A., Muller, W. J., Zar, H. J., Brooks, D., Grenham, A., Hamrén, U. W., Mankad, V. S., Ren, P., Takas, T., Abram, M. E., Leach, A., Griffin, M. P., & Villafana, T. (2022). Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. New England Journal of Medicine. https://doi.org/10.1056/NEJMoa2110275
Jones, J. M. (2023). Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR. Morbidity and Mortality Weekly Report, 72. https://doi.org/10.15585/mmwr.mm7234a4
McNulty, R. (2025, November 14). Real-World Data Show Preventive Nirsevimab Reduces Infant RSV Hospitalizations | AJMC. https://www.ajmc.com/view/real-world-data-show-preventive-nirsevimab-reduces-infant-rsv-hospitalizations
Payne, A. B., Battan-Wraith, S., Rowley, E. A. K., Stockwell, M. S., Tartof, S. Y., Dascomb, K., Irving, S. A., Dixon, B., Ball, S. W., Tenforde, M. W., Vazquez-Benitez, G., Stephens, A. B., Han, J., Natarajan, K., Salas, S. B., Bezi, C., Sy, L. S., Lewin, B., Sheffield, T., … Link-Gelles, R. (2025). Effectiveness of nirsevimab among infants in their first RSV season in the United States, October 2023–March 2024: A test-negative design analysis. Lancet Regional Health - Americas, 49, 101196. https://doi.org/10.1016/j.lana.2025.101196
RSV Immunizations: Two Ways to Protect Babies. (2025, October 10). HealthyChildren.Org. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/RSV-immunizations-new-ways-to-protect-babies.aspx
Sumsuzzman, D. M., Wang, Z., Langley, J. M., & Moghadas, S. M. (2025). Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: A systematic review and meta-analysis. The Lancet Child & Adolescent Health, 9(6), 393–403. https://doi.org/10.1016/S2352-4642(25)00093-8



